Catalytic Generation of Superoxide by Different Alcohols
DOI:
https://doi.org/10.5530/fra.2019.1.8Keywords:
Alcohols, Superoxide, Redox Cycling, Chemiluminescence, Reduced Glutathione (GSH), Sulfur, SeleniumAbstract
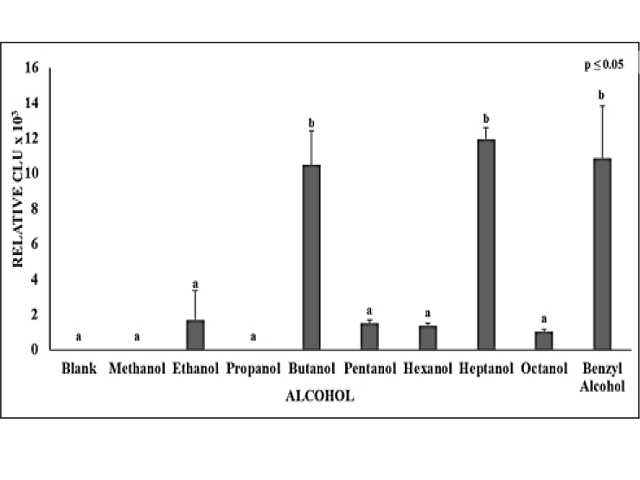
Objective: Group VIA elements, oxygen, sulfur and selenium can be toxic and many of their compounds are toxic to cells owing to the catalytic generation of superoxide and oxidative stress from thiol oxidations. Sulfides, (RS-) and selenides, (RSe-) of organic molecules and enzymes are often redox catalysts. In the current study, alcohols (ROH) were investigated to ascertain if oxides (RO-) of some alcohols might also ionize and redox cycle generating superoxide. Methods: The Lucigenin chemiluminescence assay was used for the detection of superoxide generation by the aliphatic alcohols and Benzyl Alcohol at 25°C and 37°C in the presence or absence of reduced glutathione (GSH). Similar Benzyl compounds of sulfur, selenium and oxygen were also tested for direct comparison of their catalytic activity. Results: Many of the alcohols tested, generated superoxide in the presence of GSH at both 25°C and 37°C, but not in the absence of GSH. Overall catalytic activity was greater at 37°C than at 25°C. Comparing the catalytic activity of equal concentrations of the S, Se and O moiety of the Benzyl compounds showed that although the catalytic alcohols did generate superoxide in the presence of GSH, but the sulfur and selenium compounds showed greater catalytic activity. Conclusion: As hypothesized, some aliphatic alcohols tested did generate superoxide similar to many sulfur and selenium analog compounds in the presence of GSH. From the results we can deduce that some alcohols may be following a redox mechanism that is similar to the S and Se compounds that redox cycle in presence of GSH generating superoxide.
Downloads
Metrics